GMO-FREE EUROPE Conference
Since the first genetically modified organisms (GMOs) were approved for cultivation in Europe, GMO-FREE EUROPE conferences have brought together farmers, activists, politicians, entrepreneurs and scientists.
Since 2003, we have successfully coordinated resistance to the release of GMOs into the environment and exchanged information and experiences. We defend our right to farm and produce food without genetic engineering for the sake of the protection of biodiversity, freedom of choice and our regional sovereignty.
In 2023, the European Commission proposed to deregulate the authorisation and deliberate release of new GMOs into the environment. Without risk assessment, labelling and traceability, they would be impossible to control or avoid. We are firmly opposed to such deregulation in an era of rapidly expanding technical possibilities for so-called gene editing.
We defend GMO-free agriculture and food production and seek to work with like-minded partners around the world.



Past conferences
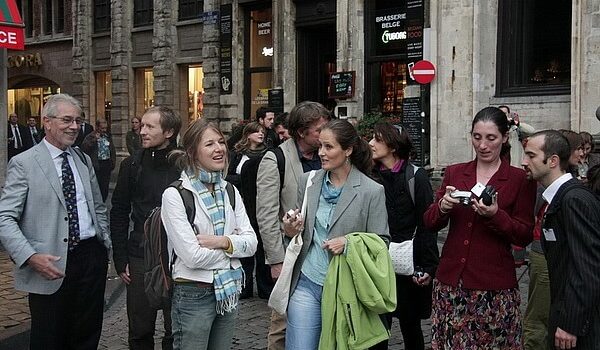
2022 | GMO Free Europe Event

2018 | GMO Free Europe Conference

2015 | GMO-FREE EUROPE

 Spain
Spain Czechia
Czechia France
France Portugal
Portugal Resources
Resources Austria
Austria